Lewis number is defined as the ratio of thermal diffusivity to mass diffusivity. The driving force of the heat is the temperature difference. Similarly, mass diffusion is caused due to concentration difference (the dominant driving force in mass diffusion is concentration). In convective heat and mass transfer, the effect of boundary layer formation is an important phenomenon. There are three boundary layer types. They are,
- Velocity boundary layer
- Thermal boundary layer and
- Concentration boundary layer
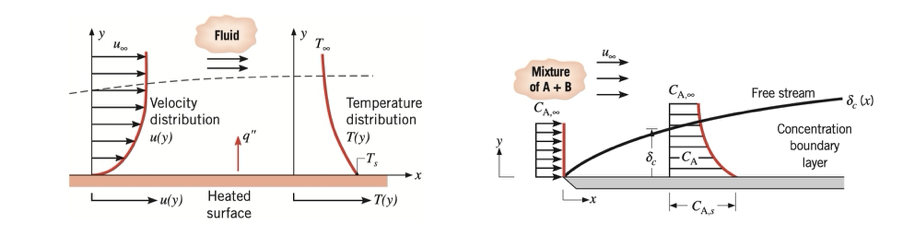
When both mass transfer and heat transfer occur simultaneously by diffusion, the concept of Lewis number (Le) is important. It is a dimensionless number and it compares the relative size of the thermal boundary layer to the concentration boundary layer.
Variations of Lewis Number
Based on the Lewis number value, we can consider three types of fluid flows. They are,
- less than one,
- equal to one or
- greater than one.
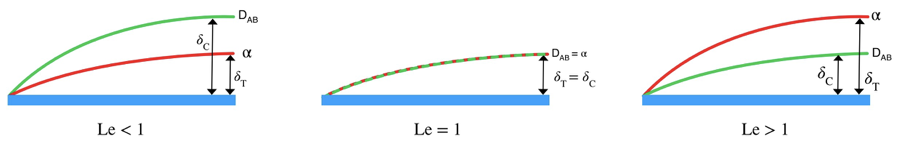
We will consider an example to further understand the situation. Let’s consider a fluid stream passing a flat plate. In figure 2.1, concentration and thermal boundary layer thickness are shown for three cases.
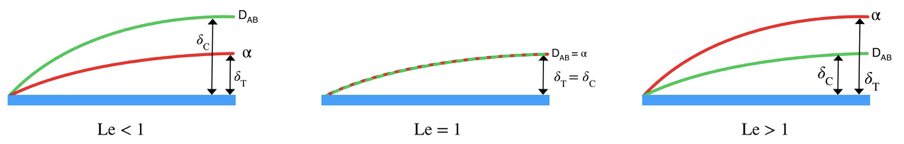
When Le < 1
In the first of the figure, the Concentration boundary layer thickness is higher than the thermal boundary layer thickness. This implies, the concentration difference is quite significant and there has been a great deal of mass diffusion. Hence the Mass diffusivity is quite high. Also, smaller temperature boundary layer thickness implies low-temperature difference and comparatively little amount of heat has diffused through the plate. Hence thermal diffusivity is quite low. So, in the first situation, the ratio between the two diffusivities becomes less than 1.
This means the fluid efficiently transfers mass than heat.
When Le = 1
In the second figure concentration boundary and thermal boundary, thickness is equal. Hence the Lewis number is exactly 1. This implies the same thermal diffusivity and mass diffusivity.
This means the fluid transfers mass and heat equally.
When Le > 1
In the third figure, the thermal boundary layer thickness is higher than the concentration boundary layer thickness. This implies higher thermal diffusivity than mass diffusivity. Hence ratio between two diffusivities becomes greater than 1.
This means the exact fluid transfer heat than mass
Lewis Number Definition
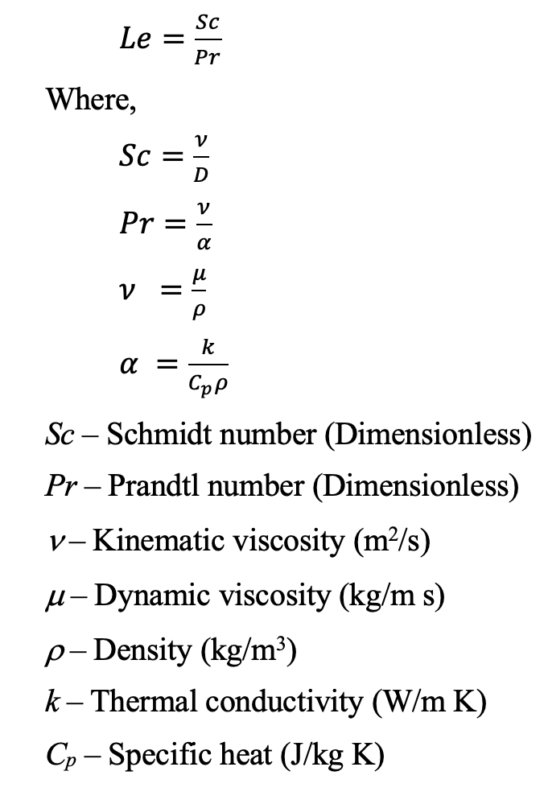
Lewis number is defined as the ratio of thermal diffusivity to mass diffusivity.
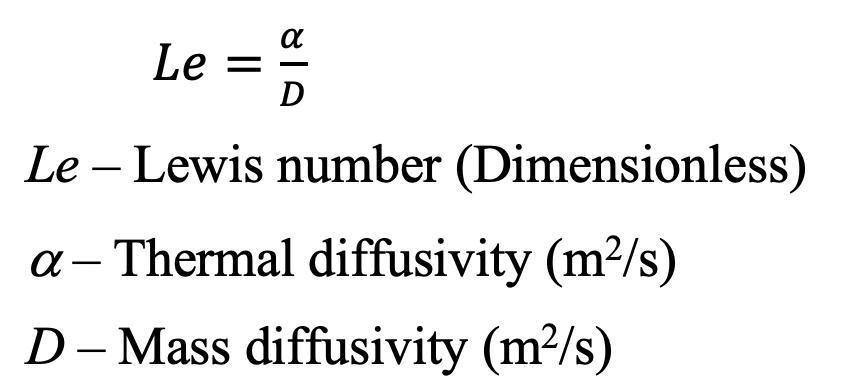
The Lewis number can be represented in several ways. It has a close relationship with the Schmidt number (Sc) and the Prandtl number (Pr).
Importance of Lewis Number
Lewis number is one of the most important things considered in the field of combustion. In this section, we will investigate the relationship between combustion and the Lewis number.
Combustion and Lewis Number
Every fluid has a Lewis number, and it has a significant impact on the combustion processes. Premixed Combustion refers to the combustion process that follows right after a mixing process of different types of reactants. So, there are several reactants in the mixture which have different Lewis numbers. Premixed combustion is used in gas turbines, spark-ignition engines, etc. Therefore, the Optimum ratio of reactants needs to be determined to proceed with efficient combustion. It helps to give minimum emissions and it helps the environment in a positive manner.
Flame speed refers to the rate of the flame front expansion in a combustion reaction. Lewis number has a big role to play in the flame speed of a combustion reaction. This is accompanied by the thermal diffusivity instability of the process. Thermal instability is an occurrence that takes place in the flame to disturb the combustion reaction. It is essential to maintain thermal stability and flame speed for an efficient and safer combustion process.
When Le > 1
Let’s consider the situation Lewis number exceeding 1. Here the thermal diffusivity is higher than mass diffusivity. Hence the heat transfer from the flame is significant whereas mass transfer into the flame isn’t that significant. This will result in a stabilized combustion.
When Le < 1
Once the number drops below 1 thermal diffusivity is lesser than that of mass diffusivity. Hence there is a probability to become the flame unstable due to the drop in the temperature and burning velocity. Very low Lewis numbers will result in incomplete combustion that can lead to greenhouse & toxic gas emissions.
When Le = 1
In this case, both thermal diffusion and mass diffusion become equal, and the combustion is a balanced one.