Introduction
Industrialization has led to thousands of factories. These Factories with complex processes generate Wastewater. Therefore, wastewater from the factories has very high BOD & COD levels. Hence, Wastewater treatment is very much essential in achieving discharge limits.
- Wastewater treatment can be classified as
- Primary treatment,
- Secondary Treatment &
- Tertiary treatment
- Primary Treatment includes Screening, Chemical Treatment &, etc.
- During Secondary treatment, Wastewater is treated Biologically.
- Tertiary treatment refers to Media Filtration (i.e -Sand, Carbon), Membrane Filtration & Disinfection.
The Biological Wastewater treatment process utilizes microorganisms like bacteria, fungi & algae to degrade Organic matter N, P-containing Substances. Aerobic, Anerarobic & Anoxic are the three common methods.
Biodegradability
Biodegradability refers to the ability of decomposing complex chemical structures of organic matter containing in wastewater to simple structures in the means of microorganisms. There are certain effluents that can be degraded by microorganisms. Some might easily convert into CO2, H2O, NH3 &, etc. while some might degrade partially. Similarly, there are types of matter which cannot be degraded by microorganisms. Biodegradability is quite vital in designing Biological wastewater treatment processes.
There are few experimental methods to identify the waste water biodegradability.
- BOD/COD Index
- Here the comparison of BOD/COD will provide an idea of the biodegradability. A very low value of the index means that effluent has a very high chemical Oxygen demand with a very low Biological oxygen demand. Hence the biodegradability will be very low. When the index falls under 0.3, effluent is hardly degradable. When the index falls between 0.3 – 0.5 effluent is biodegradable. Anything over 0.5 will be easily degradable.
- Respiratory Rate
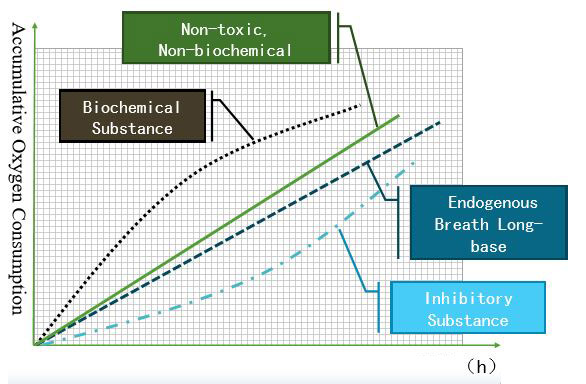
- The respiration of microorganisms mainly occurs in two different ways. Biochemical respiration is the process of degrading the organic compounds of the influent. Endogenous respiration is the process of oxidizing their own cells by microorganisms. Anyway, a system is prone self oxidize some portion of cells. But, when the microorganisms lack organic matter or the system contains non-biodegradable matter, endogenous respiration becomes predominant. Hence, the number of microorganisms decreases. When the Biochemical respiration line is well above the Endogenous respiration line, substances in the wastewater are easily biodegradable. Closer the gap between the lines, the lesser the biodegradation capacity. Once the endogenous respiration line overtakes the biochemical respiration line, substances become non-biodegradable. All these respiration rates can be measured using a Warburg-type manometer. The diagram below illustrates the biodegradability.
Aerobic Wastewater Treatment
Here the biochemical reactions take place in the presence of adequate oxygen levels.
- There are 3 major reactions in the Aerobic process. They are,
- Decomposition,
- Synthesis &
- Endogenous respiration.
- However, all these reactions are interconnected and there is no exact order of process.
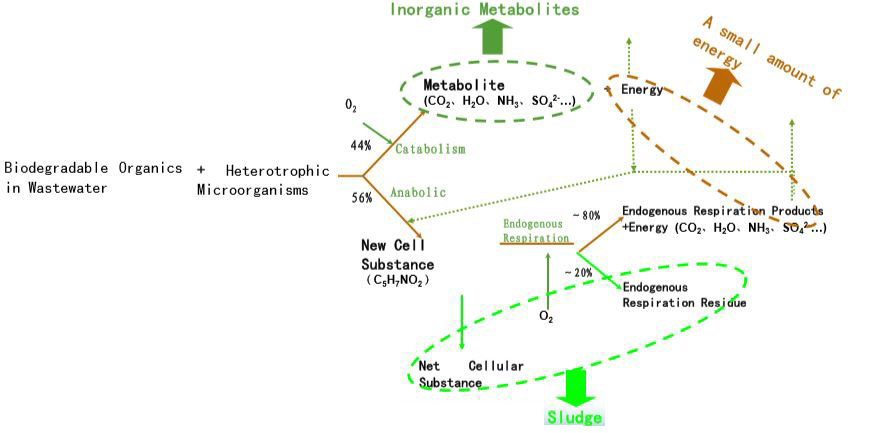
- Decomposition
- This is also called Oxidation, Metabolism & Catabolism. Here the Organic matter decomposes into CO2, H2O, NH3, etc by the Aerobic microorganisms in the presence of dissolved oxygen. In addition to the above process will generate a considerable amount of energy. Around 45% of the organic matter will decompose in the reaction.
CHONS (Organic Matter) + O2 ➜ CO2 + H2O + NH3 + SO42- + Energy
- This is also called Oxidation, Metabolism & Catabolism. Here the Organic matter decomposes into CO2, H2O, NH3, etc by the Aerobic microorganisms in the presence of dissolved oxygen. In addition to the above process will generate a considerable amount of energy. Around 45% of the organic matter will decompose in the reaction.
- Synthesis
- This is called Anabolism, Assimilation Metabolism. The remaining 60% of the Organic matter left in the first reaction is utilized to synthesize new cells of microorganisms. During the process, energy generated from Decomposition is used.
C, H, O, N, S (Organic Matter) + Energy ➜ C5H7NO2 (New Cells)
- This is called Anabolism, Assimilation Metabolism. The remaining 60% of the Organic matter left in the first reaction is utilized to synthesize new cells of microorganisms. During the process, energy generated from Decomposition is used.
- Endogenous Respiration
- C5H7NO2 + O2 ➜ CO2 + H2O + NH3 + SO42- + Energy
Few Cells will eventually self-oxidize and decompose to CO2, H2O, NH3 &, etc. Here C5H7NO2 refers to the general formula of a microorganism cell. However, some cells may contain Elements like Phosphorous & Sulphur.
Hence Formulas like,
C5H7NO2, C60H87O23N12P, C5H7O2NP0.06S0.1 will also be there to represent bacteria.
- C5H7NO2 + O2 ➜ CO2 + H2O + NH3 + SO42- + Energy
The bellow diagram provides a very clear image of these interconnected processes in Aerobic wastewater degradation.
Factors Effecting the aerobic Process
There are certain factors affecting the biological oxidation process.
- Dissolved Oxygen
- Dissolved oxygen (DO) is one of the most important factors. Simply because decomposition, as well as endogenous reparation, requires oxygen. A concentration of 1 -2 ppm of dissolved oxygen is vital in the biological oxidization process.
- Dissolved oxygen (DO) is one of the most important factors. Simply because decomposition, as well as endogenous reparation, requires oxygen. A concentration of 1 -2 ppm of dissolved oxygen is vital in the biological oxidization process.
- Temperature
- As a rule of thumb reaction rate will become double when the temperature increases by 10 C. Still, a temperature around 15-40 C is ideal for the reaction processes. Any temperature above this limit will result in negative reaction rates. Proteins & Acids containing in the cells are very sensitive to temperature. Hence, sudden temperature rises will affect the process greatly.
- As a rule of thumb reaction rate will become double when the temperature increases by 10 C. Still, a temperature around 15-40 C is ideal for the reaction processes. Any temperature above this limit will result in negative reaction rates. Proteins & Acids containing in the cells are very sensitive to temperature. Hence, sudden temperature rises will affect the process greatly.
- Nutrients
- As per the general formulas of microorganisms around 97% of the composition is by C, H, O & N. Remaining 3% will be from P, S & metal elements. Considering the type of effluent there are a few occasions where nutrients like N, P, and Metals must be added. For Aerobic Wastewater treatment a ratio of 100: 5: 1 of BOD: N: P is generally maintained.
- As per the general formulas of microorganisms around 97% of the composition is by C, H, O & N. Remaining 3% will be from P, S & metal elements. Considering the type of effluent there are a few occasions where nutrients like N, P, and Metals must be added. For Aerobic Wastewater treatment a ratio of 100: 5: 1 of BOD: N: P is generally maintained.
- pH value
- Proper care must be given to the pH of the system. Sometimes even the microbial activities tend to affect the pH value. The optimum pH range for the aerobic Biological process is 6.5 – 8.5. Lower pH will result in fungal dominance and sludge bulking.
- Proper care must be given to the pH of the system. Sometimes even the microbial activities tend to affect the pH value. The optimum pH range for the aerobic Biological process is 6.5 – 8.5. Lower pH will result in fungal dominance and sludge bulking.
- Organic Loading Rate
- Organic loading rate refers to kilograms of BOD of organic matter loaded to unit weight of microorganisms per unit time (i.e. –kgBOD/ (kgVSS.day). When the loading rate exceeds the affordable limit of microorganisms, desired reactions will not take place.
- Organic loading rate refers to kilograms of BOD of organic matter loaded to unit weight of microorganisms per unit time (i.e. –kgBOD/ (kgVSS.day). When the loading rate exceeds the affordable limit of microorganisms, desired reactions will not take place.
- Toxic Substance
- Toxic matter such as Halogens, Heavy metals, H2S, alcohol & Cyanides will inhibit the reactions of the microorganisms. Further, these toxic substances will result in dead cells which will completely cease the treatment process.
Anaerobic Wastewater Treatment
Unlike in Aerobic treatment, Microorganisms under anaerobic (No Oxygen) or even facultative (Less Oxygen) conditions decompose organic matter will decompose mainly into CO2 & CH4. The end product of the process is biogas. This process is also called anaerobic digestion & anaerobic fermentation. The process can be explained by two-stage, three-stage, or four-stage theory. The four-stage theory explains the whole picture of the treatment process.
Four Stage Theory
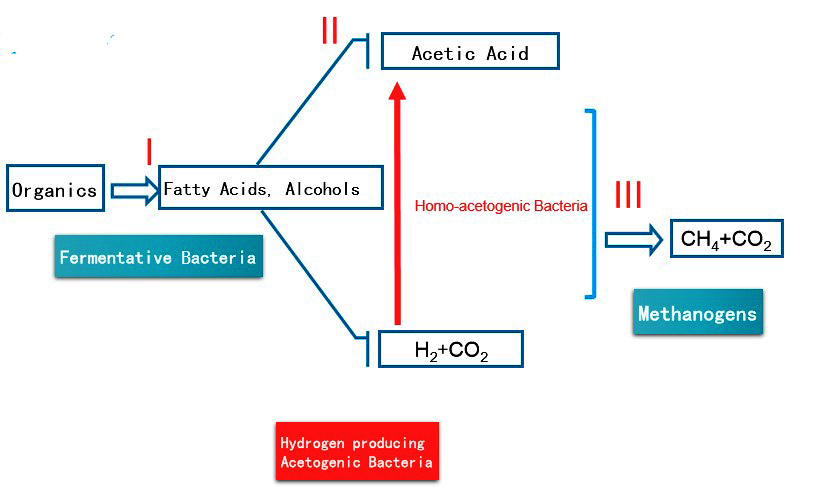
Stage 1
- Here Fermentative bacteria is the active microorganism that in the process. The main functions in the stage are Hydrolysis & Acidification.
- First complex insoluble organics will turn into soluble organic compounds through hydrolysis bacteria.
- Thereafter, Acidification bacteria transforms these soluble matters into Fatty Acids and Alcohols.
- Although the Hydrolysis process is time-consuming, acidification happens at a rapid rate. Therefore hydrolysis is the limiting factor in stage 1.
Insoluble Organics ➜ Soluble matter (Hydrolysis)
Soluble matter ➜ Fatty Acids + Alcohlos (Acidification)
Stage 2
- Here Hydrogen producing bacteria is the active microorganism. The main function in the stage is to convert high molecular weight fatty acids & alcohols into acetic acid & hydrogen.
- It is quite important to make sure that all fatty acids & alcohols are decomposed in this process. The leftover will no longer decompose in the following steps.
Fatty acids & Alcohols ➜ Acetic Acid + H2 (Acetogensis)
Stage 3
- This is an intermediate process that contributes very little to the end process. Here Homo Acetogenic bacteria converts a very little portion of Hydrogen formed in stage 2 into Acetic acid.
- Compared to Acetic acid generated in stage 2 this portion from stage 3 is almost negligible.
H2 + CO2 ➜ Acetic Acid (Homo Acetogenis)
Stage 4
- The main function of the process is to decompose Acetic Acid & H2 into Methane & CO2. This is also the final stage of the process.
- There are two main bacteria in the process.
- Acetate – trophic methanogens decompose Acetic Acid into CH4 & CO2. This process contributes to around 70% of CH4 production.
- Hydrogen tropic methanogens convert H2 & CO2 into CH4 which accounts for the remaining 30%.
- In addition to these decompositions, methanogens will also form CH4 from the residuals like Formic acid, Methanol, Methyl Sulphide & Tri Methyl Amine.
Acetic Acid + H2 + CO2 ➜ CH4 + CO2 (Methanogenis)
Bellow diagram illustrates the whole procedure in the Anaerobic digestion.
Factors Effecting Anaerobic Digestion
There are certain factors affecting the Digestion process
- Temperature
- For Mesophilic bacteria, the ideal temperature will be around 35 C. whereas thermophilic bacteria prefer a temperature around 55 C.
- As the temperature rises reaction rate will increase. When the temperature reaches the maximum affordable value reaction rate will start to drop significantly.
- pH value
- pH is one of the most important factors in anaerobic digestion. 6.5 -7.5 will be the ideal range for the process.
- In addition to controlling the pH of the effluent entering the digester, pH variations inside the reactor must be considered. The acetogenic process results in increasing the pH while methanogenic results in decreasing pH.
- Methanogenic bacteria are very sensitive to sudden pH variations. Hence pH controlling becomes a vital part of anaerobic digestion.
- Nutrients
- Although the N, P requirement is less compared to the aerobic process, a ratio of 400: 5: 1 of COD: N: P is ideal for the process.
- Although the N, P requirement is less compared to the aerobic process, a ratio of 400: 5: 1 of COD: N: P is ideal for the process.
- Organic Loading Rate
- The anaerobic reaction has the ability to absorb very high organic loading rates compared to aerobic conditions. Typically it can increase even up to 50 COD/ (m3. Day).
- Though the reaction rate in acetogenins is quite high, Methonogenis has a very low reaction rate. Hence controlling loading rates is vital and the deciding reaction for loading rate will be Methanogenis. Optimization of Organic loading rate will result in low hydraulic retention time & low volume of the reactor.
- Toxic Substance
- Toxic matter such as Halogens, Heavy metals H2S alcohol & Cyanides will inhibit the reactions of the microorganisms. Further, these toxic substances will result in dead cells which will completely cease the treatment process.
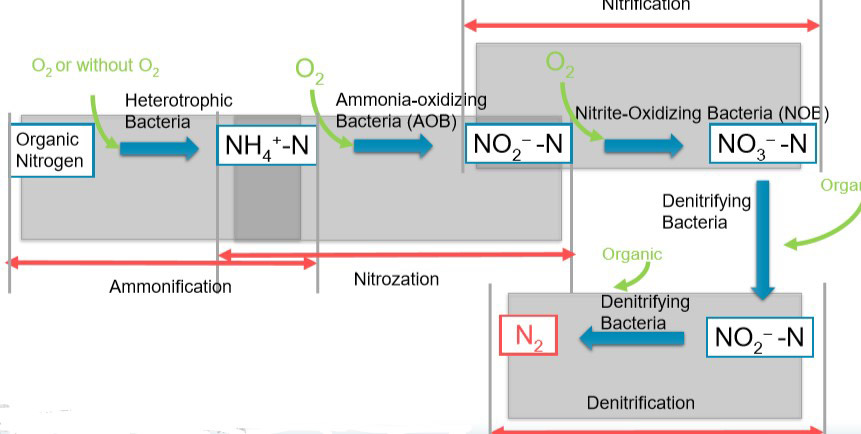
Biological Nitrogen Removal Process
- The nitrogen removal process from organic matter comprises three major steps. They are,
- Ammonification,
- Nitrification &
- Denitrification.
- Ammonification
- Nitrogen-containing in organic matter easily converts into Ammonium Nitrogen (NH4+-N). This is a quite simple process & doesn’t need extra effort.
- This will happen in either aerobic conditions or anaerobic conditions.
- Nitrification
- It is the process of converting Ammonium Nitrogen into Nitrite and then followed by converting nitrite into Nitrogen. These are Aerobic autotrophic bacteria that do not need organic matter to grow up. The carbon source for the bacteria is organic Carbon & the energy source is Nitrogen compounds in the wastewater.
NH4+ + 1.5 O2 ➜ NO2– + H2O + 2 H+ (Nitrification)
NO2– + 0.5 O2 ➜ NO3– (Nitration) - Ideally, a dissolved Oxygen level of over 1ppm, Temperature around 25-30 C & a pH around 8 – 8.5 is essential for the process.
- It is better to maintain a low BOD value (i.e. 20 ppm) because too much BOD will result in a competition for dissolved Oxygen between Nitrogen and Organic matter.
- It is the process of converting Ammonium Nitrogen into Nitrite and then followed by converting nitrite into Nitrogen. These are Aerobic autotrophic bacteria that do not need organic matter to grow up. The carbon source for the bacteria is organic Carbon & the energy source is Nitrogen compounds in the wastewater.
- Denitrification
- It is the process of reducing Nitrite & Nitrite under anaerobic conditions with the aid of Denitrifying bacteria.
- Here Nitrates and Nitrites will act as electron acceptors & Organic matter will act as electron donors.
- Firstly NO3– turns into NO2– & then NO2– turns into NO–. Finally NO– will turn into N2 to release N from the effluent.
- The system should have an adequate carbon source to act as electron donors. Necessary action must be taken when the Carbon levels decrease, by adding compounds like Methanol.
- Should maintain maximum possible anaerobic conditions. Denitrification prefers temperatures between 20 -30 C and pH values of 6.5 – 7.5.
Bellow diagram illustrates the Denitrification process.
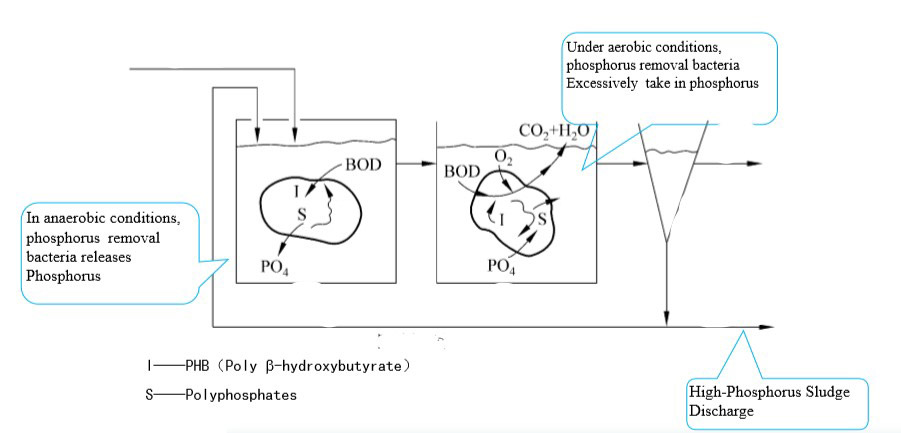
Biological Phosphorus Removal Process
Most of the Microorganisms in the activated sludge process will absorb some portion of phosphorous which helps to synthesize its cell. In addition to this, there is a bacteria called phosphobacteria which has the ability to intake Phosphorous than its requirement. They will store Phosphorous by a means of polymerization. Phosphate will store as an energy source.
In the Aerobic process, Bacteria will gain energy through Oxidation. This energy can be utilized to absorb Phosphates in the wastewater. After the aeration Bacteria pass to the sedimentation tank. Here a portion of bacteria is discharged as sludge. Hence the high amount of phosphate leaves the system as sludge. In order to proceed with a continuous phosphorous removal, an anaerobic unit should be there prior to aeration. Recycle sludge from the clarifier passes to the anaerobic tank. Here Phosphorus removing bacteria release phosphorous. As a result, these bacteria gain energy to absorb molecules from wastewater streams entering the anaerobic tank.
Now the Phopobacteria will enter the aeration tank with zero phosphorous residual in it. Hence it has the ability to absorb Phosphorous to its full potential. This process continues in a cyclic manner.
Below Diagram illustrates the Phoporous removal process.